 
Chemistry: Matter and ChangeChapter 17:
Reaction RatesProblem of the Week <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/ch17_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/ch17_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | It's
Luminating
The first colonists on Mars probably will be plants. Professor Rob Ferl of the University of Florida is bioengineering mustard plants for a proposed mission that will put plants on Mars as soon as 2007. Instead of altering the plants so they can adapt more easily to the conditions on Mars, Professor Ferl is adding a gene from the jellyfish that allows the plant to bioluminesce. The added gene will act as a reporter gene so the mustard plants can send messages back to Earth about how they are surviving on the planet. These plants will be genetically wired to glow with a soft green aura when they encounter problems. By glowing, the mustard plants will report low oxygen levels, low water, or poor nutrients in the soil. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> | | Bioluminescence
and chemiluminescence comes from energy released from a chemical
reaction in the form of cool light. Generally, chemiluminescence
occurs when the product of an exothermic reaction is formed
in the excited state. As the electron returns to the lower ground
state, it releases energy that can be seen as a photon of light.
Excited intermediates produced during a chemical reaction do
not have favorable pathways to release this energy. When the
intermediate species encounters a fluorescer it will transfer
the energy. The fluorescer acts as a catalyst for the decomposition
of the key intermediate, and this catalyst is an important factor
in the efficiency of this chemical reaction. Luminol is a common
fluorescer found in chemiluminescence reactions. One of the
commonly known chemiluminescence phenomena that is visible to
the human eye and occurs in living organisms is the bioluminescence
of fireflies and photobacteria. In a luminescent reaction, two
types of chemicals, luciferin and luciferase, combine together.
The luciferase acts as an enzyme, allowing the luciferin to
release energy as it is oxidized. The color of the light depends
on the chemical structures of the chemicals. GFP, green fluorescent
protein, is a fluorescent protein isolated from coelenterates,
such as the pacific jellyfish. Aequorin, a bioluminescent photoprotein
isolated from jellyfish is activated by calcium ions, and emits
a blue light of 470nm. Due to its high sensitivity to Ca2+,
aequorin has been widely used an indicator of intercellular
calcium concentrations. | | | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | The following is the reaction mechanism used by many organisms that bioluminesce.
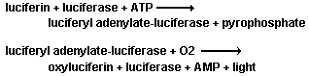 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_3.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_3.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a>
a. Identify the intermediates and the catalyst in this
reaction.
b. Determine the complex reaction.
c. Draw the reaction energy diagram for this exothermic
reaction. | | | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
The following is the reaction mechanism for the chemiluminescence
reaction of bis(2,4-dinitrophenyl) oxalate with hydrogen peroxide
and 9, 10, 11, 12-tetraphenyl napthacene:
C14H6N4O12+ H2O2 → C6H5OC2O4H + C6H5OH + products
C6H5OC2O4H → C2O4 + C6H5OH
C2O4 + C42H28 → CO2 + [CO2] · - + [C42H28]· +
[CO2]·-+ [C42H28]· + → CO2 + [C42H28]*
[C42H28]* → hv (light) + C42H28
From the above reaction mechanism, identify the intermediates, identify the catalyst, and determine the complex reaction. | | | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
A light stick is made of a flexible plastic tube containing
a thin walled glass tube. The glass tube contains hydrogen peroxide
dissolved in a phthalic ester. The plastic tube contains a phenyl
oxalate ester and a fluorescent dye. The complex reaction and
reaction mechanism for the light stick are given.
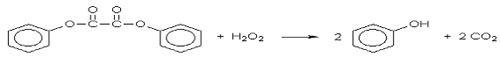 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (6.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch17_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (6.0K)</a>
oxalate ester + H2O2 → I + 2
phenol + 2 CO2
I + fluorophor (F) → F* + products
F* → F + hv(light)
a. Using the above reaction mechanism, determine the
intermediates and the catalyst in this chemiluminescent reaction.
b. Explain what a catalyst does in the reaction. Draw
a reaction energy diagram to represent the addition of a catalyst.
|  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> | Useful Web Sites:
Chemiluminescence
The Chemiluminescence Home Page
Major Luciferin Types
Lecture 1 - Introduction
Bioluminescence Questions and Answers
Leafy Green Astronauts
Energy and Metabolism
Chemiluminescence of oxalate esters
Chemiluminescence: The Chemistry of Making Light
Chemiluminescent History Complex Plastics, Inc.
All Omniglow Product Markets | |
 |  |
|





