 
Chemistry: Concepts and ApplicationsChapter 3:
Introduction to the Periodic TableProblem of the Week
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch03_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch03_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWsolutions.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWsolutions.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | Teachers Notes:
Problem 1:
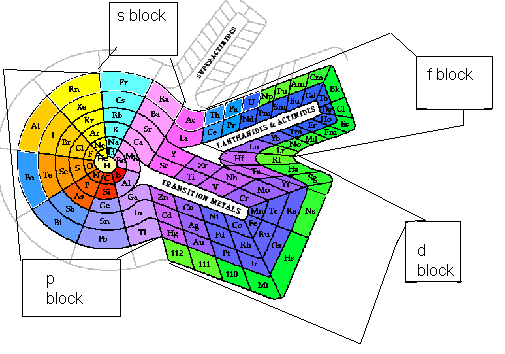 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/Image47.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (17.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/Image47.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (17.0K)</a>
| Problem 2:
On each spoke of the spiral, the atomic radii increases, the ionization energy decreases, and the electronegativity decreases. As one travels from hydrogen, clockwise around the spiral, the atomic radii decreases until the same color is reached. The ionization energy increases until the same color is reached. The electronegativity increases until the same color is reached.
| Problem 3:
Element 118 would fall in the yellow region of the table. The electron configuration would be ns2np6. It should have an ionization energy less that 10.7 and an atomic radii greater than 1.34 Angstrom.
| Extension: Have students design there own periodic table. A possible Web site to use: http://www.genesismission.org/educate/scimodule/cosmic/ptable.html. Fun sites to review symbols:
http://www.acs.org/vc2/11pz/oct96.pdf
http://www.acs.org/vc2/11pz/Oct97.pdf
|
 |  |
|





