 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch17_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch17_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWsolutions.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWsolutions.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
Teachers Notes: Problem 1:
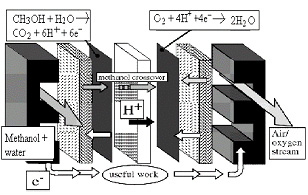
a. Anode Cathode
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078617987/179001/ch21_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (38.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078617987/179001/ch21_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (38.0K)</a>
b. Anode reaction: CH3OH + H2O → CO2 + 6 H+ + 6 e-
Cathode reaction: O2 + 4 H+ + 4 e- → 2 H2O
c. Since the electrons lost must equal the electrons
gained
2(CH3OH + H2O → CO2 + 6 H+ + 6 e--) and
3(O2 + 4 H+ + 4 e- → 2 H2O)
2CH3OH + 2H2O → 2CO2 + 12 H+ + 12 e-
3O2 + 12 H+ + 12 e- → 6 H2O
Overall reaction: 2CH3OH + 3O2→ 2CO2 + 4H2O which can
be simplified to:
CH3OH + 3/2O2→ CO22 + 2 H2O
|





