 
Chemistry: Matter and ChangeChapter 22:
HydrocarbonsProblem of the Week <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/ch22_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/ch22_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | Cracking
Hydrocarbons
Petroleum has been used by many different civilizations throughout history. The Egyptians used pitch, a tar residue, to seal pyramids and coat mummies, while ancient Babylonians used pitch to pave their roads and hold building stones together. Bitumen, thick black oil, was used in Mesopotamia to caulk ships and set stones in jewelry. In North American, Native Americans used crude oil as body paint and fuel for ceremonial fires. Crude oil is found in many areas of the world and was successfully drilled from the earth in 1859 by Col. Edward Drake in Titusville, Pennsylvania. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (26.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (26.0K)</a> |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (25.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (25.0K)</a> | Crude oil is a complex mixture of hydrocarbons classified by the carbon-hydrogen ratio. To obtain the greatest number of useful components, crude oil must be refined. The first refinery opened in 1861 producing kerosene, a fuel that replaced whale oil in oil lamps. For 30 years kerosene was the most important petroleum product, but the invention of both the light bulb and the internal combustion engine changed the direction of refineries. The demand for kerosene decreased, while the need for gasoline and diesel fuel increased. Modern refineries wash crude oil with water to remove impurities such as salt. Fractional distillation is used to separate the light crudes, small molecules with low carbon-hydrogen ratios, from heavy crudes. Heavy crudes include many large molecules that are unused. These unused fractions are cycled through a cracking unit to break them down into more useful components. | | The
process of cracking hydrocarbons was developed in 1913 to provide
more valuable and useful hydrocarbons to the consumer. Cracking
is accomplished by different methods in the cracking unit of
a petroleum refinery. Catalysts are used to speed up the cracking
reactions in catalytic cracking. Common catalysts are zeolite,
bauxite, aluminum hydrosilicate and silica-alumina. In the absence
of a catalyst, thermal cracking occurs. Thermal cracking occurs
when heavy crudes are heated to high temperatures often under
high pressures to break the bonds in these larger molecules
forming smaller more usable hydrocarbons. Steam cracking is
a method that involves the use of high temperature steam to
break smaller alkanes and form alkenes. The cracking process
involves complex reactions that covert large complex hydrocarbons
into smaller hydrocarbons that have lower boiling points. Hydrogen
gas may also be a product of the cracking process.
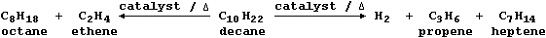 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_4.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078664187/179001/ch22_4.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a>
|  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | For each of the equations, refer to the web sites referenced
below. Then, write and draw the structural formula of each
molecule represented.
a. Cracking propane to yield propene and hydrogen
b. Cracking normal heptane to produce propane and 1-butene
c. Cracking decane to produce pentane and 2-pentene | | | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
Dodecene, C12H24, is obtained from the catalytic cracking of
hexadecane, C16H34. Draw and name 3 isomers of dodecene. Include
a structural isomer and a geometric isomer. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078664187/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> | Useful Web Sites:
Chemistry of Thermal Cracking
Thermal Cracking Processes
Petroleum and Coal
Chemistry of Petroleum Cracking
Case Study: Petroleum
Organic Chemistry | |
 |  |
|





