Chemistry: Concepts and ApplicationsChapter 3:
Introduction to the Periodic TableProblem of the Week <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch03_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/ch03_chapter.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | Electrons and Atoms—The Periodic Table
Although many elements had been known since ancient times, the first scientific discovery of an element occurred in 1669 when Hennig Brand discovered phosphorus.
Since that time, the list of known elements and their properties has continued to grow. Many scientists have attempted to organize this information. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078617987/179001/ch6_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (29.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078617987/179001/ch6_1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (29.0K)</a> | | In 1829, German chemist Johann Döbereiner took a first step toward the statement of the periodic law and the development
of the periodic table by stating the law of triads. Döbereiner noted the existence of three-element groups, called triads, in which the atomic mass and the properties of the middle
element were averages of the other two elements. Döbereiner’s triads included calcium, strontium, and barium, and chlorine, bromine, and iodine.
At about the same time that Newlands formulated his law of octaves in the early 1860s, the French geologist A.E. Béguyer de Chancourtois wrapped a list of the elements arranged
by increasing atomic mass around a cylinder. He called this arrangement of the elements the telluric screw. Elements with similar properties, such as oxygen, sulfur, selenium, and tellurium,
fell into vertical columns. | While writing his textbook Principles of Chemistry, Dmitri Mendeleev created a card containing the symbol, atomic mass, and the chemical and physical properties for each of the
63 known elements. Mendeleev laid the cards out in various organizational patterns until he grouped elements with similar properties in vertical columns. Mendeleev developed his statement
of the periodic law based on the patterns observed in his table. Although Mendeleev’s periodic table is over 130 years old, it resembles the periodic tables that are in the today’s chemistry classroom. Modern periodic tables arrange the elements
by increasing atomic number thanks to H.G. Moseley’s work around 1913. Glenn T. Seaborg made the last major change to the periodic table when he added the lanthanide series and actinide
series in the 1945 to accommodate the newly discovered transuranium elements. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWproblem_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | There are several alternate periodic table designs in use today. This spiral periodic table was presented by Professor Theodor Benfey in 1960. Locate the s, p, d, and f block elements
on the Benfey periodic table. | | | 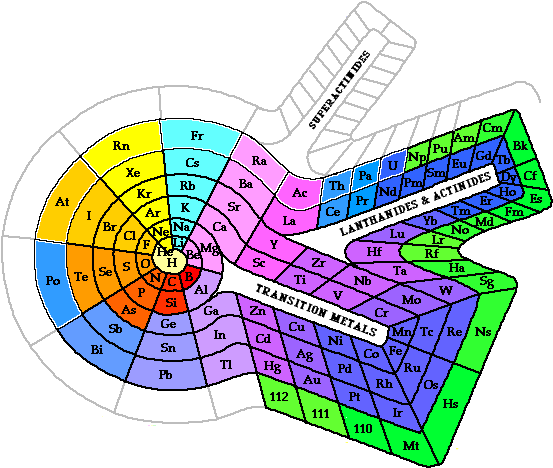 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/Image46.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (25.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/Image46.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (25.0K)</a> | | | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/POWproblem_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | Determine the trends of atomic radii, ionization energy, and electronegativity on the Benfey periodic table. | | |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::POWproblem_3::/sites/dl/free/0078617987/179001/POWproblem_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif">POWproblem_3 (0.0K)</a>POWproblem_3 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::POWproblem_3::/sites/dl/free/0078617987/179001/POWproblem_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif">POWproblem_3 (0.0K)</a>POWproblem_3 | Predict the properties of element 118. |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0078617987/179001/webLinks.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> | Useful Web Sites:
A Periodic Table of the Elements
Periodic Tables
Periodic Trends
An Early History of LBNL | |
 |  |
|